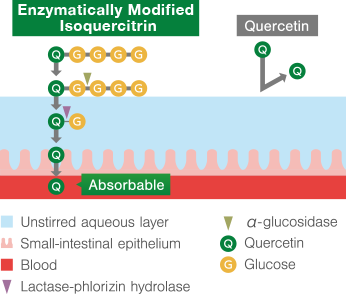
Enzymatically Modified Isoquercitrin
in Supplements

Features of Enzymatically Modified Isoquercitrin

Safety of Enzymatically Modified Isoquercitrin
The safety of Enzymatically Modified Isoquercitrin has been found safe in many toxicity studies and has been self-affirmed as Generally Recognized as Safe (GRAS) by FDA (GRAS Notice No.GRN000220).
Available (as functional material)
U.S., Canada, Japan
Functions of Enzymatically Modified Isoquercitrin
Blood flow improvement
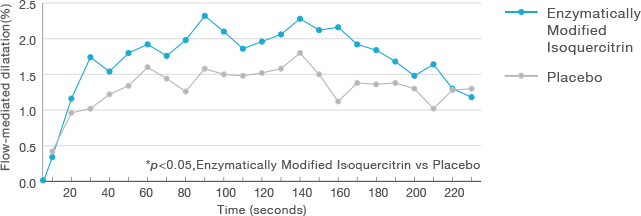
Intake of Enzymatically Modified Isoquercitrin can improve vascular function.
Dose:Enzymatically Modified Isoquercitrin 293 mg/60 kg body weight
Subjects:25 volunteers at risk of cardiovascular disease
Administration period:Single
Evaluation: FMD (Flow-mediated dilatation)
<K. Croft et al., Br. J. Nutri., 20, 1-8, 2019, https://doi.org/10.1017/S0007114519002137>
Improvement of FMD
Anti-allergies
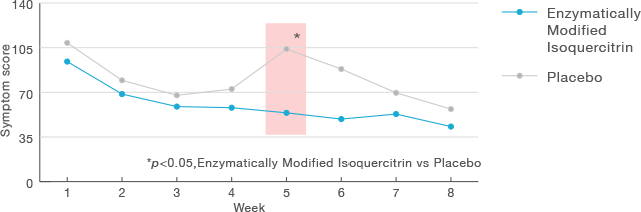
Intake of Enzymatically Modified Isoquercitrin can reduce the hay fever symptoms.
Dose: Enzymatically Modified Isoquercitrin 100 mg/day
Subjects: 20 volunteers with Japanese cedar pollinosis
Administration period: 8 weeks
Evaluation:Symptom score (sneezing, runny nose, nasal obstruction,
lacrimation, redness and itching of the eyes)
<M. Kawai et al., Int. Arch. Allegy Immunol., 149, 359-368, 2009, https://doi.org/10.1159/000205582>
Improvement of the symptom score
Stimulates saliva secretion
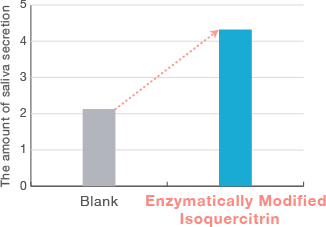
Intake of chewing gum containing Enzymatically Modified Isoquercitrin can promote
salivation.
Dose: Chewing gum containing 1.4 mg Enzymatically Modified Isoquercitrin
Subjects:3 healty volunteers
Administration period:Single
Evaluation:The amount of saliva secretion
<Patent filing:WO2017/094910 A1 >
Increase in saliva secretion
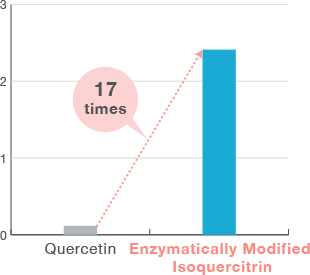
High bioavailability
In humans, Enzymatically Modified Isoquercitrin is 17 times more bioavailable than quercetin.
<Modified from K. Murota, et al., Arch. of Biophysics, 501, 91-97, 2010, https://doi.org/10.1016/j.abb.2010.06.036>
Plasma concentration(µM)
Absorption mechanism of Enzymatically Modified Isoquercitrin